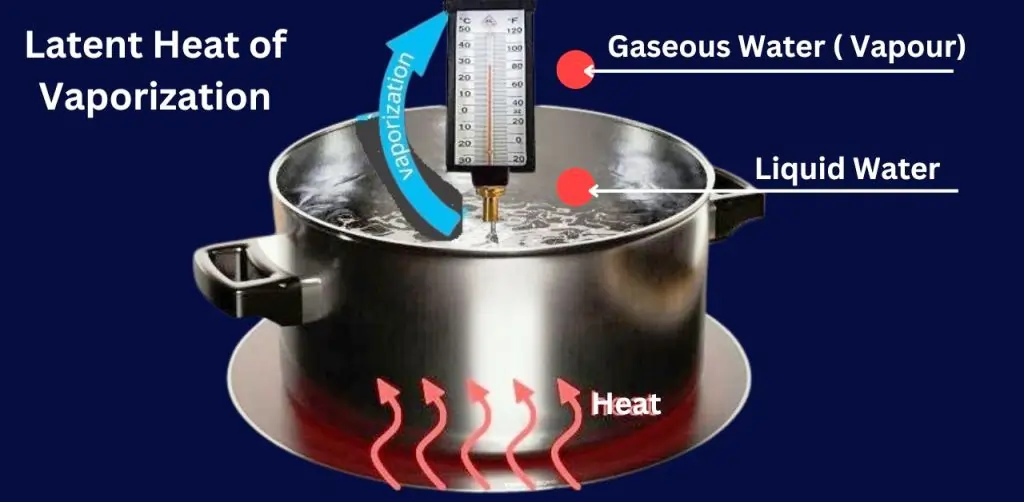
Latent Heat of Vaporization
Latent heat of vaporization refers to the amount of heat required to change a liquid into a vapor without changing its temperature. This fundamental thermodynamic concept plays a critical role in various engineering applications, particularly in refrigeration, HVAC, and steam generation systems. Understanding latent heat of vaporization helps engineers design efficient systems by leveraging phase change processes to transfer heat more effectively, reducing energy consumption and improving system performance.
In this guide, we’ll explore the definition and working principles of latent heat of vaporization, along with its key applications in engineering. We’ll also cover the important formulas involved and how different factors like pressure and temperature impact this process. By the end, you’ll have a solid grasp of how mastering this concept can lead to improved energy efficiency and better system design in both industrial and commercial applications.
What is Latent Heat of Vaporization?
Latent heat of vaporization is the amount of heat energy required to transform a liquid into a vapor at a constant temperature and pressure, without changing the liquid’s temperature. This phase change process occurs when the liquid absorbs enough energy to overcome the molecular bonds holding it together, transitioning into the gaseous state.
In this thermodynamic process, while the liquid absorbs heat, its temperature remains constant during vaporization, with all the absorbed energy going into breaking molecular bonds. For example, water requires 2,260 kJ/kg of energy to vaporize at 100°C, while maintaining that temperature.
The relationship between pressure, temperature, and phase change is crucial. As pressure increases, the temperature at which vaporization occurs also rises, meaning more energy is required to achieve the phase transition. Conversely, under lower pressure, the boiling point decreases, requiring less heat to vaporize the liquid. This interplay directly affects the efficiency of systems such as refrigeration and HVAC.
Key Applications of Latent Heat of Vaporization in Engineering
- Refrigeration and Air Conditioning Systems
In refrigeration and air conditioning, latent heat of vaporization is a fundamental part of the refrigerant cycle. When the refrigerant absorbs heat in the evaporator, it undergoes a phase change from liquid to vapor without increasing in temperature. This vaporization process removes heat from the surrounding environment, enabling effective cooling. By maximizing the latent heat of the refrigerant, systems can operate more efficiently, improving overall cooling performance and reducing energy consumption. - Boilers and Steam Generation
In steam generation systems, such as boilers, latent heat of vaporization is critical for converting water into steam. This phase transition occurs when water reaches its boiling point and absorbs energy without a rise in temperature. Steam, which contains high latent heat, is then used for power generation or heating. Proper control and utilization of this energy during vaporization ensure efficient steam production and optimized boiler performance. - Evaporative Cooling Systems
Evaporative cooling leverages latent heat of vaporization to cool air by evaporating water. As water vaporizes, it absorbs heat from the surrounding air, reducing the air temperature. This process is widely used in HVAC systems, particularly in hot, dry climates, to enhance cooling performance while consuming less energy compared to traditional refrigeration methods. This application maximizes the use of latent heat to deliver energy-efficient cooling.
Formulas Related to Latent Heat of Vaporization
The formula for calculating the latent heat of vaporization is:

Explanation of Each Variable:
- Heat Energy (Q):
This is the total amount of energy required to change the phase of the substance from liquid to vapor at constant temperature. It’s measured in Joules (J). In real-world applications, this energy is supplied to systems like refrigeration units, steam boilers, or evaporative cooling systems. - Mass (m):
This is the mass of the liquid being vaporized, measured in kilograms (kg). In engineering applications, larger systems like industrial boilers deal with greater masses of liquid, requiring more energy for vaporization. - Latent Heat of Vaporization (L):
This is a material-specific constant representing the energy needed to vaporize 1 kilogram of the liquid without temperature change. It is measured in Joules per kilogram (J/kg). For example, water has a latent heat of vaporization of approximately 2,260 kJ/kg at standard atmospheric pressure.
Example for Clarity:
Consider 2 kg of water needing to be vaporized at 100°C. The latent heat of vaporization for water is 2,260 kJ/kg.
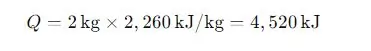
This means 4,520 kJ of heat energy is required to completely vaporize 2 kg of water.
Factors Affecting Latent Heat of Vaporization
- Pressure
Pressure plays a significant role in determining the vaporization point of a substance. As pressure increases, the temperature at which the substance vaporizes also rises, requiring more energy to convert the liquid into vapor. This is commonly observed in pressurized boilers where water boils at a higher temperature under increased pressure. Conversely, reducing pressure lowers the vaporization temperature, which is exploited in vacuum distillation processes to vaporize substances at lower temperatures and with less energy. - Temperature
Temperature directly influences the latent heat of vaporization. As temperature increases, the latent heat required for vaporization typically decreases because the liquid molecules are already at a higher energy state. For example, refrigerants in a refrigeration cycle operate at varying temperatures, and the latent heat required for vaporization adjusts accordingly. Systems designed for specific temperature ranges must account for these changes to maintain efficiency. - Substance Properties
Different substances have unique latent heat values due to their molecular structure. For example, water has a high latent heat of vaporization (~2,260 kJ/kg), making it highly effective for applications like steam generation. In contrast, ammonia and modern refrigerants like R-134a have much lower latent heat values, which are optimized for energy-efficient cooling. Understanding the specific properties of a substance helps engineers select the right material for applications like refrigeration, HVAC, and industrial processes, balancing energy use and system performance.
Significance of Latent Heat of Vaporization in Energy Efficiency
Understanding latent heat of vaporization is crucial for optimizing energy usage in various engineering systems such as refrigeration, boilers, and power plants. This concept allows engineers to effectively manage the phase change process, minimizing energy waste while maximizing system performance.
- Optimizing Energy Usage
In refrigeration systems, latent heat enables efficient cooling by allowing refrigerants to absorb large amounts of heat during vaporization without a temperature increase. This process reduces the load on compressors, leading to lower energy consumption. Similarly, in boilers and power plants, controlling the phase transition from liquid to steam ensures that energy is utilized efficiently, maximizing heat transfer and reducing fuel consumption. - Reducing Energy Consumption through Phase Change
By leveraging the phase change process, engineers can design systems that require less energy to achieve the same results. For example, in steam generation, water’s high latent heat allows the system to store and transport large amounts of energy with minimal temperature fluctuation. This efficiency reduces the overall energy input required for processes like heating or cooling, lowering operating costs. - Case Studies on Improved Efficiency
Case studies in industries such as HVAC and power generation show significant improvements in energy efficiency when latent heat is properly utilized. For example, advancements in evaporative cooling technologies demonstrate how phase change reduces energy usage by as much as 30%, while modern steam turbines achieve higher output with less fuel by precisely managing latent heat in the vaporization phase. These real-world examples underline the critical role of latent heat in achieving energy-efficient designs.
Latent Heat of Vaporization and Its Role in Modern Engineering Technologies
Latent Heat of Vaporization is the amount of energy required to convert a substance from a liquid to a vapor without changing its temperature. This fundamental thermodynamic principle plays a pivotal role in modern engineering, particularly in the development of sustainable technologies.
Impact on Sustainable Refrigerants and Eco-Friendly Cooling Systems
Latent heat is a critical factor in designing refrigerants and cooling systems. Engineers are now focusing on creating sustainable refrigerants with lower Global Warming Potential (GWP), enhancing the efficiency of cooling systems by optimizing the latent heat absorption process. These innovations reduce energy consumption, contributing to environmentally friendly solutions in industries and residential applications.
Innovations in Heat Exchangers, Steam Turbines, and Heat Recovery Systems
Latent heat is extensively utilized in various industrial processes. Advanced heat exchangers leverage latent heat to improve energy transfer efficiency, while modern steam turbines use this principle to enhance power generation. Furthermore, industrial heat recovery systems are increasingly adopting latent heat technologies to recycle waste heat, making processes more energy-efficient and reducing overall carbon footprints.
Future Trends in Engineering
The future of engineering lies in maximizing the utilization of latent heat for system performance improvements. Trends point toward the integration of latent heat into cutting-edge technologies, such as phase-change materials in energy storage, advanced HVAC systems, and optimized heat management in renewable energy systems. These developments aim to further improve efficiency and sustainability across multiple industries.
Latent heat of vaporization will continue to be a cornerstone in designing more efficient, eco-friendly systems, leading the path to a sustainable engineering future.
Conclusion
In summary, the latent heat of vaporization is an essential principle driving innovation across a wide range of engineering applications. From sustainable refrigerants and eco-friendly cooling systems to advanced heat exchangers and steam turbines, mastering this thermodynamic concept is crucial for enhancing both energy efficiency and system performance.
By fully understanding and utilizing latent heat, engineers can develop more reliable, efficient, and environmentally friendly systems. This not only optimizes industrial processes but also plays a significant role in reducing energy consumption and carbon emissions, paving the way for a more sustainable future across various industries.
FAQ on “Latent Heat of Vaporization”
Q: What is latent heat in simple words?
A: Latent heat is the energy needed to change a substance’s state without changing its temperature.
Q: What is the latent heat of vaporization of water in J/kg?
A: The latent heat of vaporization of water is approximately 2,260,000 J/kg.
Q: How to find latent heat of vaporization?
A: Latent heat of vaporization is found using the formula Q=mL, where L is the latent heat and mmm is the mass.
Q: What is the specific heat of ice?
A: The specific heat of ice is about 2,090 J/kg°C.